Avian mycoplasmosis, is a contagious respiratory disease that affects poultry, particularly chickens and turkeys. It is caused by various Mycoplasma spp. and can lead to significant economic losses in the poultry industry.  Mycoplasmosis is primarily caused by two species of Mycoplasma: Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS). These bacteria are responsible for a range of respiratory and systemic infections in birds. These are simplest prokaryotic microorganisms with the ability of self-replication, the most distinguishing characteristic of these bacteria is lack of cell wall. MG is the most common cause of mycoplasmosis in poultry and infected chicken shows a wide variety of symptoms including rales, coughing, nasal discharge, conjunctivitis, reduced feed efficiency, air sacculitis and egg production typically declines with initial infection, then recovers and maintained at a lower level.
Mycoplasmosis is primarily caused by two species of Mycoplasma: Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS). These bacteria are responsible for a range of respiratory and systemic infections in birds. These are simplest prokaryotic microorganisms with the ability of self-replication, the most distinguishing characteristic of these bacteria is lack of cell wall. MG is the most common cause of mycoplasmosis in poultry and infected chicken shows a wide variety of symptoms including rales, coughing, nasal discharge, conjunctivitis, reduced feed efficiency, air sacculitis and egg production typically declines with initial infection, then recovers and maintained at a lower level.
 Mycoplasma gallisepticum infection in birds usually takes a long time to manifest and is followed by severe respiratory tract inflammation. In flocks, the disease often goes unnoticed and causes latent infections. According to earlier studies, conditions including high feed density, heat and cold stress, high levels of ammonia, accumulation of feces, fouling of the chicken house, wide temperature variations, and abrupt climatic changes can all contribute to the spread and outbreak of disease.
Mycoplasma gallisepticum infection in birds usually takes a long time to manifest and is followed by severe respiratory tract inflammation. In flocks, the disease often goes unnoticed and causes latent infections. According to earlier studies, conditions including high feed density, heat and cold stress, high levels of ammonia, accumulation of feces, fouling of the chicken house, wide temperature variations, and abrupt climatic changes can all contribute to the spread and outbreak of disease.
Virulence factors
Variety of surface polypeptides and lipoproteins of MG plasma membrane are known and have putative function in motility, cytadhesion, surface antigen variation, and nutrient acquisition, all of which are essential virulence factors for MG.
Host- Pathogen interaction
M. gallisepticum attaches itself to the ciliated cells in the respiratory tract of birds using specialized surface proteins. For effective colonization and eventual pathogenesis, the attachment of MG to host cell is utmost important. Once attached, it invades the host cells. The gliding motility of Mycoplasma gallisepticum organisms enables them to enter target tissues and overcome host’s physical defences such respiratory mucus and ciliary activity.
Colonization and Multiplication: Numerous MG cytadhesins and putative cytadhesins have a recognized role in emergence phenotypic variation, which is assumed to be a key virulence component since it appears to facilitate chronic infection and evasion of host immune response. After invading the cells, the bacterium replicates within them. This leads to damage to the host cells, interfering with their normal function.
Inflammatory response: The host’s immune system recognizes the presence of the bacterium and mounts an inflammatory response. This involves the release of various immune cells and inflammatory mediators to combat the infection.
Tissue Damage and Lesions: The inflammation, coupled with the bacterial replication and host immune responses, can lead to damage in the respiratory tissues. This damage may manifest as lesions in the trachea, bronchi, and air sacs.
Transmission
M. gallisepticum can spread within a flock through respiratory secretions, faeces, and contaminated equipment or environment. Some wild bird species, multiage commercial layer flocks, and backyard flocks are potential MG infection reservoirs. The upper respiratory tract and conjunctiva are portals of entry for organism in aerosols or droplets.
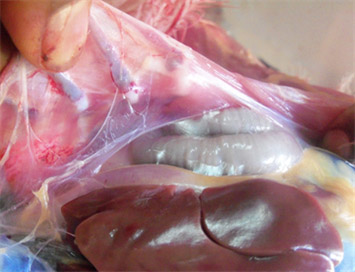 The dynamics of MG infection relies heavily on clinically or sub clinically infected carrier birds since Mycoplasma gallisepticum hardly survives outside the host for longer than a few days, yet several studies reported the ability of MG to survive up to several days on contaminated fomite materials (dust, feathers etc) provides important insights on epidemiology of the disease. Some M. gallisepticum strains capacity to develop biofilms may enable them to survive in the environment for longer duration. MG can be transmitted from infected breeder flock to their progeny via transovarian transmission. Some studies concluded that the vertical transmission of MG occurs at the highest rates during the acute phase of disease when the level of MG is at peak in respiratory tract and declines subsequently as the post infection interval lengthens.
The dynamics of MG infection relies heavily on clinically or sub clinically infected carrier birds since Mycoplasma gallisepticum hardly survives outside the host for longer than a few days, yet several studies reported the ability of MG to survive up to several days on contaminated fomite materials (dust, feathers etc) provides important insights on epidemiology of the disease. Some M. gallisepticum strains capacity to develop biofilms may enable them to survive in the environment for longer duration. MG can be transmitted from infected breeder flock to their progeny via transovarian transmission. Some studies concluded that the vertical transmission of MG occurs at the highest rates during the acute phase of disease when the level of MG is at peak in respiratory tract and declines subsequently as the post infection interval lengthens.
Carrier State: Some birds may become carriers of M. gallisepticum, meaning they harbour the bacterium without showing overt signs of illness. These carriers can serve as a source of infection for other birds.
Symptoms
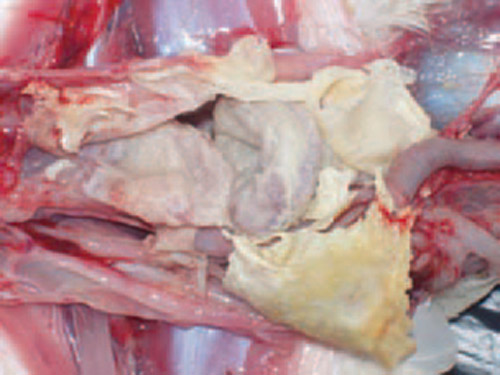 Respiratory Distress: Birds infected with mycoplasmosis often exhibit respiratory distress, characterized by coughing, sneezing, nasal discharge, and wheezing.
Respiratory Distress: Birds infected with mycoplasmosis often exhibit respiratory distress, characterized by coughing, sneezing, nasal discharge, and wheezing.
Reduced Egg Production: In laying hens, mycoplasmosis can lead to a decrease in egg production, as well as a decrease in egg quality.
Swollen Eyes and Sinusitis: Infected birds may develop swollen eyes and sinusitis, along with a discharge from the eyes.
Conjunctivitis: The infection can lead to inflammation of the conjunctiva, causing redness and discharge from the eyes.
General Weakness: Infected birds may exhibit lethargy, decreased appetite, and a drop in overall activity levels.
Diagnosis
Accurate diagnosis of mycoplasmosis is crucial for effective management. It is typically achieved through a combination of clinical signs, post-mortem examinations, and laboratory tests. These tests may include serology (blood tests), PCR (polymerase chain reaction), and bacterial isolation from affected tissues.
Prevention and Control
Biosecurity Measures: Implement strict biosecurity measures to prevent the introduction and spread of mycoplasmosis. This includes limiting visitor access, maintaining separate footwear and clothing for workers, and disinfecting equipment and facilities regularly.
Vaccination: There are vaccines available for both MG and MS, determine the most appropriate vaccination schedule for your poultry.
Cleanup Programs: Use of appropriate molecule for effective cleaning up of mycoplasmal infection prior to vaccination may provide better results.
Minimize Stress: Stress weakens the immune system, making birds more susceptible to infections. Provide a low-stress environment by ensuring proper nutrition, ventilation, and living conditions.
Surveillance: Regularly monitor your flock for any signs of illness. Early detection allows for prompt intervention and reduces the spread of the disease.
Authors:
- Dr Dushyant Pande, Product Manager- Biologicals, tech.vax@stallen.com
- Dr Sanjay Singhal, Chief Operating Officer, dr.ss@stallen.com
Reference: Beaudet, J., E. R. Tulman, K. Pflaum, X. Liao, G. F. Kutish, S. M. Szczepanek, L. K. Silbart, and S. J. Geary. 2017. Transcriptional profiling of the chicken tracheal response to virulent Mycoplasma gallisepticum strain Rlow. Infect. Immun. 85: e00343- 17. Browning, G.F., A.H. Noormohammadi, and P.F. Markham. 2014. Identification and characterization of virulence genes in mycoplasmas. In: Mollicutes – Molecular Biology and Pathogenesis. G.F. Browning and C. Citti, ed. Caister Academic Press, Norfolk, UK. 77–90. Boguslavsky, S., D. Menaker, I. Lysnyansky, T. Liu, S. Levisohn, R. Rosengarten, M. Garcia, and D. Yogev. 2000. Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect Immun. 68:3956–3964. Evan, J. D., S. A. Leigh, S. L. Branton, S. D. Collier, G. T. Pharr, and S. M. D. Bearson. 2005. Mycoplasma gallisepticum: current and developing means to control the avian pathogen. J. Appl. Poult. Res. 14:757–763. Hochachka, W. M., and A. A. Dhondt. 2000. Density-dependent decline of host abundance resulting from a new infectious disease. Proc. Natl. Acad. Sci. USA. 97:5303–5306. Indikova, I., M. Vronka, and M.P. Szostak. 2014. First identification of proteins involved in motility of Mycoplasma gallisepticum. Vet Res. 45:99. Ishfaq, M., W. Zhang, W. Hu, S. Waqas Ali Shah, Y. Liu, J. Wang, Z. Wu, I. Ahmad, and J. Li. 2019. Antagonistic effects of Baicalin on mycoplasma gallisepticum-induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infect. Drug Resist. 12:3075–3089. Jan, G., C. Brenner, and H. Wroblewski. 1996. Purification of Mycoplasma gallisepticum membrane proteins p52, p67 (pMGA), and p77 by high‐ performance liquid chromatography. Protein Expr Purif. 7:160–166. Keeler, C.L., Jr., L.L. Hnatow, P.L. Whetzel, and J.E. Dohms. 1996. Cloning and characterization of a putative cytadhesin gene (mgc1) from Mycoplasma gallisepticum. Infect Immun. 64:1541–1547. 130. Ley, D. H. 2003. Mycoplasma gallisepticum infection. Pages 722–743 in Diseases of Poultry. Y. M. Saif, ed. 11th ed. Iowa State Press, Ames, IA. Mikaelian, I., D.H. Ley, R. Claveau, M. Lemieux, and J.P. Berube. 2001. Mycoplasmosis in evening and pine grosbeaks with conjunctivitis in Quebec. J Wildl Dis. 37:826–830. Miyata, M. and D. Nakane. 2014. Gliding mechanism of the Mycoplasma pneumoniae subgroup. In: Mollicutes – Molecular Biology and Pathogenesis. G.F. Browning and C. Citti, eds. Caister Academic Press, Norfolk, UK. 237–253. Raviv, Z., S. Callison, N. Ferguson‐Noel, V. Laibinis, R. Wooten, and S.H. Kleven. 2007. The Mycoplasma gallisepticum 16S‐23S rRNA intergenic spacer region sequence as a novel tool for epizootiological studies. Avian Dis. 51:555–560. Szczepanek, S.M. and L.K. Silbart. 2014. Host immune responses to mycoplasmas. In: Mollicutes – Molecular Biology and Pathogenesis. G.F. Browning and C. Citti, eds. Caister Academic Press, Norfolk, UK. 273–288. Zimmermann, C.U. 2014. Current insights into phase and antigenic variation in mycoplasmas. In: Mollicutes – Molecular Biology and Pathogenesis. G.F. Browning, and C. Citti, eds. Caister Academic Press, Norfolk, UK. 165–196.