In an exclusive interview to Poultry TRENDS magazine, Mr. Sanjeev Khanna talks about the current biosecurity & hygiene challenges in poultry industry and his startup – Envizon Biosciences.
Mr. Sanjeev Khanna is an Entrepreneur, Leader & strategist. He has completed his UG from BHU & PG in Business Management from BYB, Mumbai. He has over 25 years of working experience with renowned MNCs like Ranbaxy, Elanco & Novus in various leadership positions with different job responsibilities & geographies. He was instrumental in establishing some of the great companies & brands in Indian subcontinent and spent most of his career span in business development in Livestock and Poultry segment. When he started his journey in Chandigarh, little did he know that this journey will be so exciting and rewarding.
Currently he is working as Managing Director, Envizon Biosciences, a startup based out in Bengaluru and focused on biosecurity & hygiene program in Indian subcontinent. He is adventurous & an explorer who loves traveling, hiking, camping to offbeat places, he is also a health freak & believes that Living a healthy life would extend longevity and regenerate the body and mind.
Excerpts of the interview:
How you describe yourself?
Team player, strategic thinker, grounded & believes in fundamentals to run the businesses & relations. As a professional I got opportunities to explore different geographies and to lead some of the great teams across sub-continent. Working with people with different mindsets and motivations was always exciting and thought provoking. Influencing teams & individuals to explore their true potential and helping them to become successful is always interesting and exciting for me. My strengths lies in people, team building & business development. I always believe that leadership is not a position or title it is an action & example We make leadership decisions every minute of every day, both at work and in our personal lives. When we speak up for what is right, either at work or in our home lives, we are demonstrating leadership.
How did you come with the idea of entering this field?
After working in different segments including Livestock & Poultry for so many years, I finally decided to take different course and started my own venture in mid 2021. I was looking for the key challenges our Industry is undergoing. What are the challenges faced by the producers and how we should provide them science based & trustworthy solutions.
I would like to quote Albert Einstein “ Relativity applies to physics, not ethics”. As in ethics either you are 100% ethical/honest or you are 0% & you cannot be in between place, same theory applies in Biosecurity, either it is 100% or 0% & there is no space in between.
After lot of research and brainstorming, we found that Biosecurity & Hygiene was one of the most over stretched words across the Industry in whole South Asian region, In spite of so many players and almost everyone talking about Biosecurity, still there are huge challenges of disease outbreaks which ultimately shrink the profitability of producers. There is no proven method to validate the claims made by different solution providers either. For example, a product selling at X price & another at 10X have almost same label claims and the producers are in a fix as to what to use and what are the value offered to them. In Envizon Biosciences we invested in machines and equipment to validate the claims to show the value of the solutions with ROI.
What is Envizon Biosciences? Where is it based at?
Envizon Biosciences is a startup company based out in Bengaluru and focused on science, technology & innovation. Our core is new conceptual products which can add value to the customers business. In 2021 Envizon Biosciences entered into an agreement with Intracare NL, a globally renowned company for biosecurity and hygiene products, for marketing of its solution in India. The combination of these leading businesses will offer greater sustainability and broader scope than either alone. In addition to Intracare’s track record of creating successful collaborations, Envizon looks forward to unlocking the shared potential of its combined business strengths with Intracare. By combining Intracare’s state-of-the-art R&D technologies & future proof solutions with Envizon’s diverse distribution network & able workforce, the joint venture will be a category leader in Biosecurity and Hygiene products. Beside that Envizon Biosciences is also working closely with other strategic business partners across the globe to bring latest technologies with good ROI to Indian producers.
What are the specific challenges or obstacles commonly faced when trying to maintain high levels of hygiene by farmers ?
As mentioned above, the biggest challenge we found is lack of right information and validation points. Also, during our research, we didn’t find right equipment and machines which is required to complete the job properly and professionally with many producers. Even with a good product available in the market, if application is not proper, it cannot produce the desired results. The staff who is applying the biosecurity products needs proper and regular training, scientific information & latest updates to use the solutions correctly. Currently many products sold in the market based on relationship & perception, instead of actual scientific data and information.
What is Intra-Hygiene concept?
As you must aware of, in the intensive livestock farming, we are constantly challenged by the threat of pathogens. If pathogens succeed to enter the farm, we are facing production and economical losses, decreased animal welfare, increased mortality, which ultimately leads to higher antibiotic usages & increase the cost. The only way to prevent entry of diseases into the farm is with optimal biosecurity. To mitigate these challenge and help producers to have ultimate biosecurity, Envizon Biosciences for the first time in India, launched a novel concept of Intra Hygiene program for Sheds, Hatcheries and Processing plants with help of latest science-based technologies.
These technologies have been launched first time in India and has two parts – one is the effective solutions, and another is its effective applications with help of the latest machines (mainly imported from Europe) and they play an important role to give full-proof results. All the program is having different validation points to share the results & ROI.
How is Intra-Hygiene different from existing products available in the market?
The current solutions available in Indian market and used by producers are of industrial grade which has been used mostly in the different industries and then started using in the poultry & livestock Industry as well. They are corrosive, not recommended in Livestock Industry and having lot of adverse effects directly or indirectly on the humans & animals, be it in livestock or poultry. All the Intra hygiene solutions used by Envizon biosciences are of food grade & European Union certified, They all are approved by top rated credentials authorities like ECHA, NSF etc. and all the solutions are highly effective with no adverse impact on livestock, machines & equipment.
How do you test the challenge level in a given poultry farm?
As I mentioned, Biosecurity is one of the most overused word across livestock & poultry Industry. People know of it in literal terms only. Washing the farms, spraying disinfectants is not completing the biosecurity cycle and that is the reason we see lot of incidences of disease outbreaks periodically. Then, the biggest challenge in our markets are validation of results.
Envizon Biosciences have a dedicated and certified team for Intra hygiene services. The team is equipped with all the latest scientific equipment to establish the difference and to validate the results before and after the services.
Explain the various stages involved in the process
There are 7 stages involved here in the process to complete the program successfully. Unless we clean the organic materials from the farm, cages, and other specific places, we can not eradicate micro-organisms from the farm. Therefore, we go with different stages including dry cleaning, wet cleaning, foam cleaning, disinfection, and validations at pre & post stages.
Are your products internationally certified? Which other countries is this product being used?
Yes, all the products are internationally certified by international credit rating companies like ECHA, NSF etc under PT01, PT02, PT03, PT04 & PT05. All the products are registered & use in all European, US & Asian countries with extremely satisfied customers. The products are completely safe for the poultry birds, on people who are handling it and on all the expensive machines used in our Industry.
What are the different areas within poultry chain where Intra-Hygiene concept can be applied?
The products can be applied in different poultry chain including Broiler, Layer, Hatcheries, setters & processing units in different dosages. There is no adverse impact of the products as all the products are human grade and safe to use in even food, chicken processing units and even in human hospitals.
What sort of equipment is used for carrying out the task? Do you have a professional team to carry out this task?
Yes, Envizon Biosciences invested in the equipment for proper applications of solutions and we have latest equipment & certified professionals across to do the job successfully.
How has been the market feedback so far? Any big names to share?
We are working on many exciting projects across India and South Asia region. Due to professional ethics & confidentiality agreements, it would not appropriate to share names.
Future depends upon the actions we take today. I do see lot of changes in the producer’s thinking process due to changes in consumer’s behaviour and demand.
India is one of the fastest growing economies in the world & home of 1.4 billion people. With so much development, fast urbanization & youth population, the demand of protein is going to be even higher in coming time. India’s 1Bn young population are well informed, well-travelled and know exactly what they need. With international exposures and social media influences, consumers are now aware and looking for food safety & hygiene while making their choices. This is going to drive our industry and will change the whole scenario and the way it works; the focus is shifting rapidly from production to the processes and safety of the food, which in return is bringing lot of opportunities to the producers to invest in food safety and concepts like Intra Hygiene to achieve their goals.
I am pretty confident that things are changing, and they are changing pretty fast for betterment of our industry.
For any queries, Mr. Khanna can be reached at sanjeev.khanna@envizon.co.in





 Mycoplasma gallisepticum infection in birds usually takes a long time to manifest and is followed by severe respiratory tract inflammation. In flocks, the disease often goes unnoticed and causes latent infections. According to earlier studies, conditions including high feed density, heat and cold stress, high levels of ammonia, accumulation of feces, fouling of the chicken house, wide temperature variations, and abrupt climatic changes can all contribute to the spread and outbreak of disease.
Mycoplasma gallisepticum infection in birds usually takes a long time to manifest and is followed by severe respiratory tract inflammation. In flocks, the disease often goes unnoticed and causes latent infections. According to earlier studies, conditions including high feed density, heat and cold stress, high levels of ammonia, accumulation of feces, fouling of the chicken house, wide temperature variations, and abrupt climatic changes can all contribute to the spread and outbreak of disease.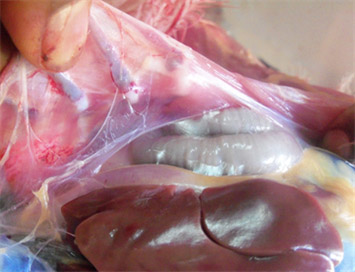 The dynamics of MG infection relies heavily on clinically or sub clinically infected carrier birds since Mycoplasma gallisepticum hardly survives outside the host for longer than a few days, yet several studies reported the ability of MG to survive up to several days on contaminated fomite materials (dust, feathers etc) provides important insights on epidemiology of the disease. Some M. gallisepticum strains capacity to develop biofilms may enable them to survive in the environment for longer duration. MG can be transmitted from infected breeder flock to their progeny via transovarian transmission. Some studies concluded that the vertical transmission of MG occurs at the highest rates during the acute phase of disease when the level of MG is at peak in respiratory tract and declines subsequently as the post infection interval lengthens.
The dynamics of MG infection relies heavily on clinically or sub clinically infected carrier birds since Mycoplasma gallisepticum hardly survives outside the host for longer than a few days, yet several studies reported the ability of MG to survive up to several days on contaminated fomite materials (dust, feathers etc) provides important insights on epidemiology of the disease. Some M. gallisepticum strains capacity to develop biofilms may enable them to survive in the environment for longer duration. MG can be transmitted from infected breeder flock to their progeny via transovarian transmission. Some studies concluded that the vertical transmission of MG occurs at the highest rates during the acute phase of disease when the level of MG is at peak in respiratory tract and declines subsequently as the post infection interval lengthens.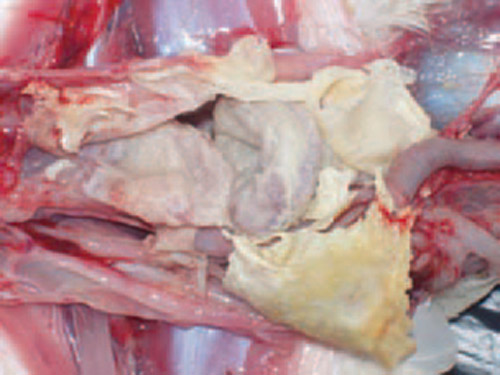 Respiratory Distress: Birds infected with mycoplasmosis often exhibit respiratory distress, characterized by coughing, sneezing, nasal discharge, and wheezing.
Respiratory Distress: Birds infected with mycoplasmosis often exhibit respiratory distress, characterized by coughing, sneezing, nasal discharge, and wheezing.